Analytics & Testing
Consumer Safety And Product Efficacy Are Our Top Priorities
End-to-End Validation That Goes Beyond Compliance

THC Compliance
Biomass Testing
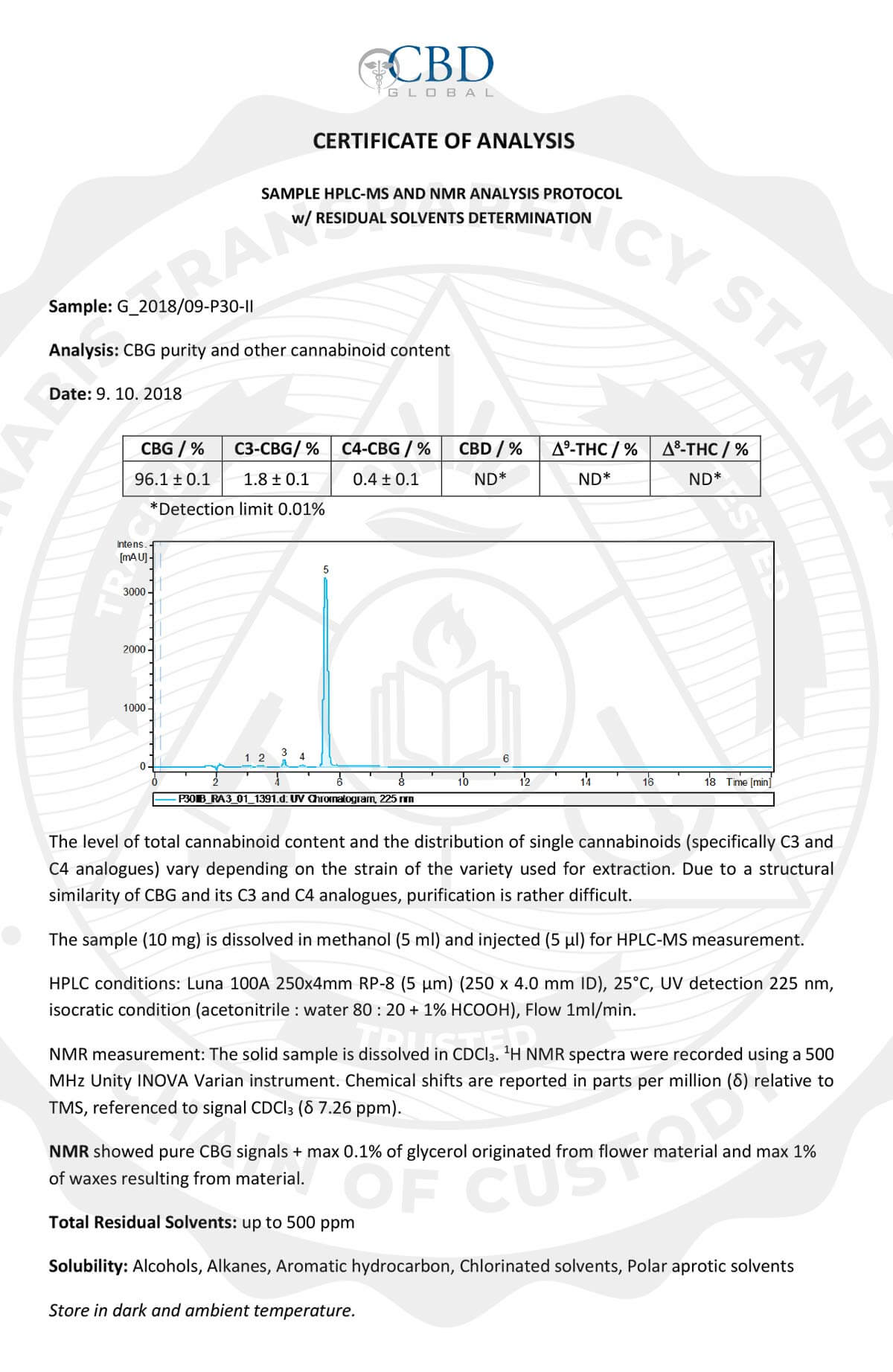
All pre-harvest and post-harvest biomass is tested to show legal adherence to THC content of less than 0.3% on a dry weight basis. After approval, the material begins lot and batch tracking, storage, and processing.
This same initial potency COA identifies all other base cannabinoid levels and relative ratios to each other, serving as a chain of custody to validate that all extracts are derived from their listed biomass source.
Potency Test Used
High-Performance Liquid Chromatography (HPLC)

Full Panel Certificates of Analysis (COA) on All Extracts
Safety & Compliance Above That Required by the FDA
POTENCY
- High Performance Liquid Chromatography (HPLC)
- Gas Chromatography (GC-FID)
- Nuclear Magnetic Resonance (NMR)
HEAVY METALS
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
RESIDUAL SOLVENTS
- Head-Space Gas Chromatography (GC-FID)
- Head-Space Mass Spectrometry (GC-MS)
TERPENE PROFILE
- Gas Chromatography (GC-MS/MS)
PESTICIDES
- Gas Chromatography (GC-MS/MS)
- Liquid Chromatography (LC-MS/MS)
- LC-MS/MS (through the “QuEChERS” method of extraction)
- High Performance Liquid Chromatography (HPLC)
- Gas Chromatography (GC-ECD)
MICROBIALS & BIOLOGICALS
- Aerobic Plate Count (APC)
- Yeast and Mold Count
- Coliforms
Our Choice of 3rd Party Labs




Third-party labs that meet CBDG’s qualification criteria must:
■ Be Drug Enforcement Agency (DEA) registered
■ Post their Standards of Deviation
■ Have more sensitive Limits of Quantitation (LOQs) than the industry average
■ Be willing to discuss their protocols and results
■ Be focused on quality over volume
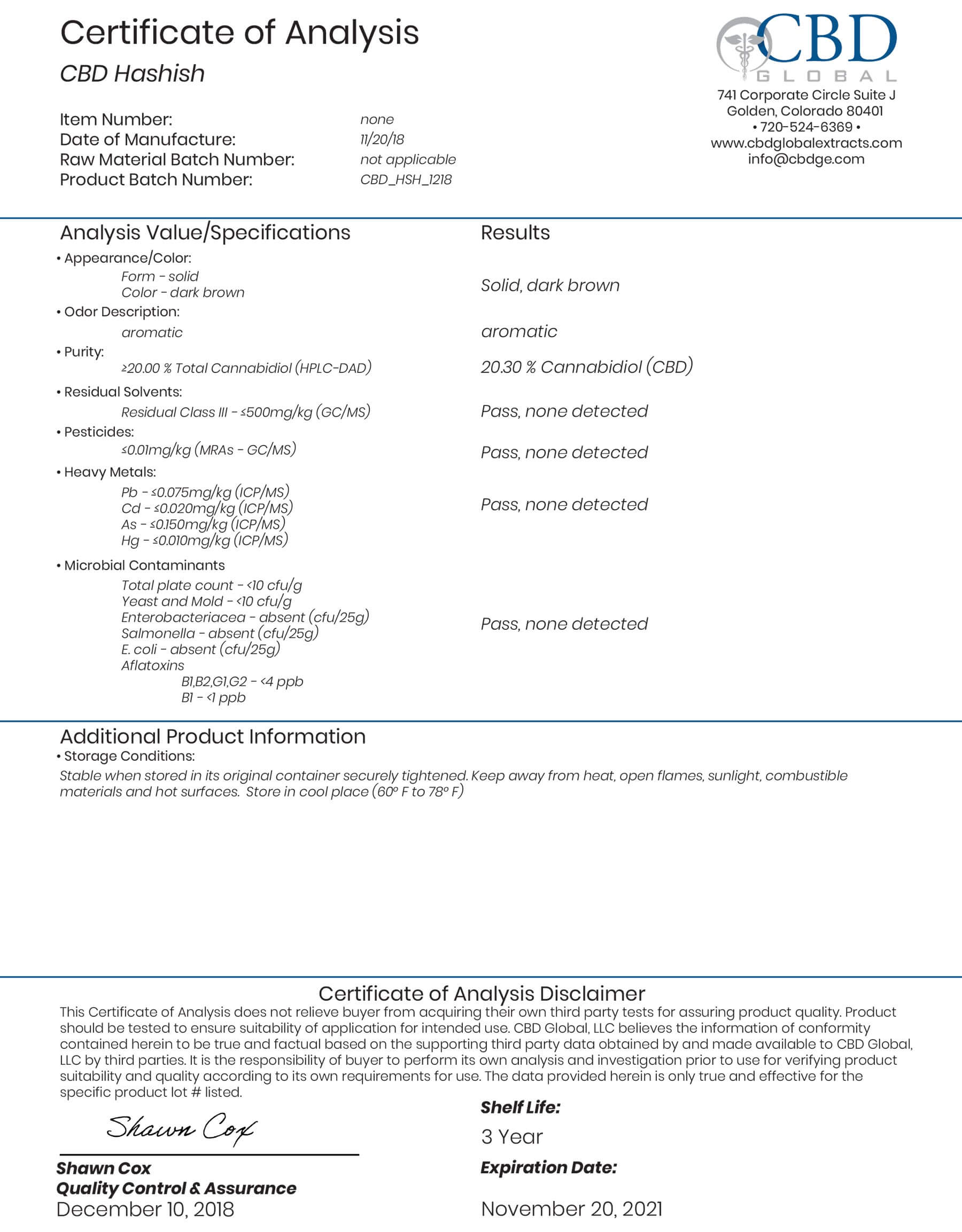
Potency Level
Tests on Final Products
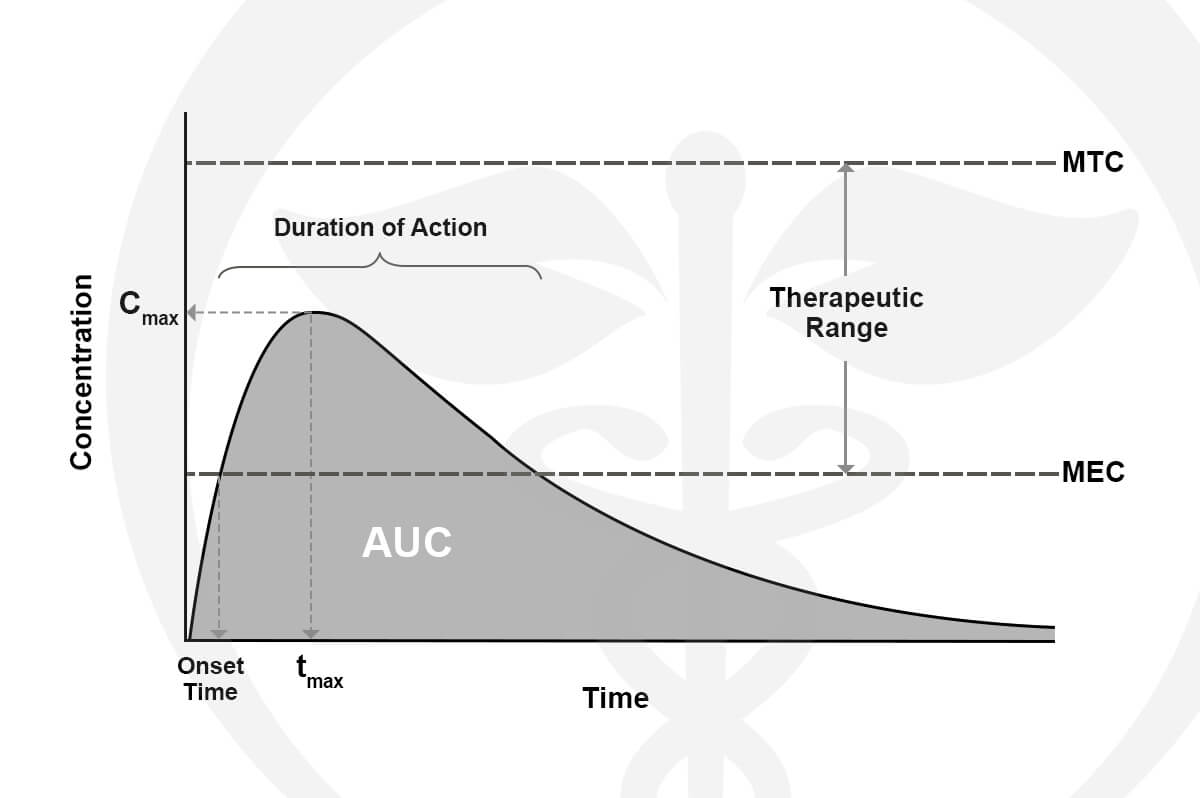
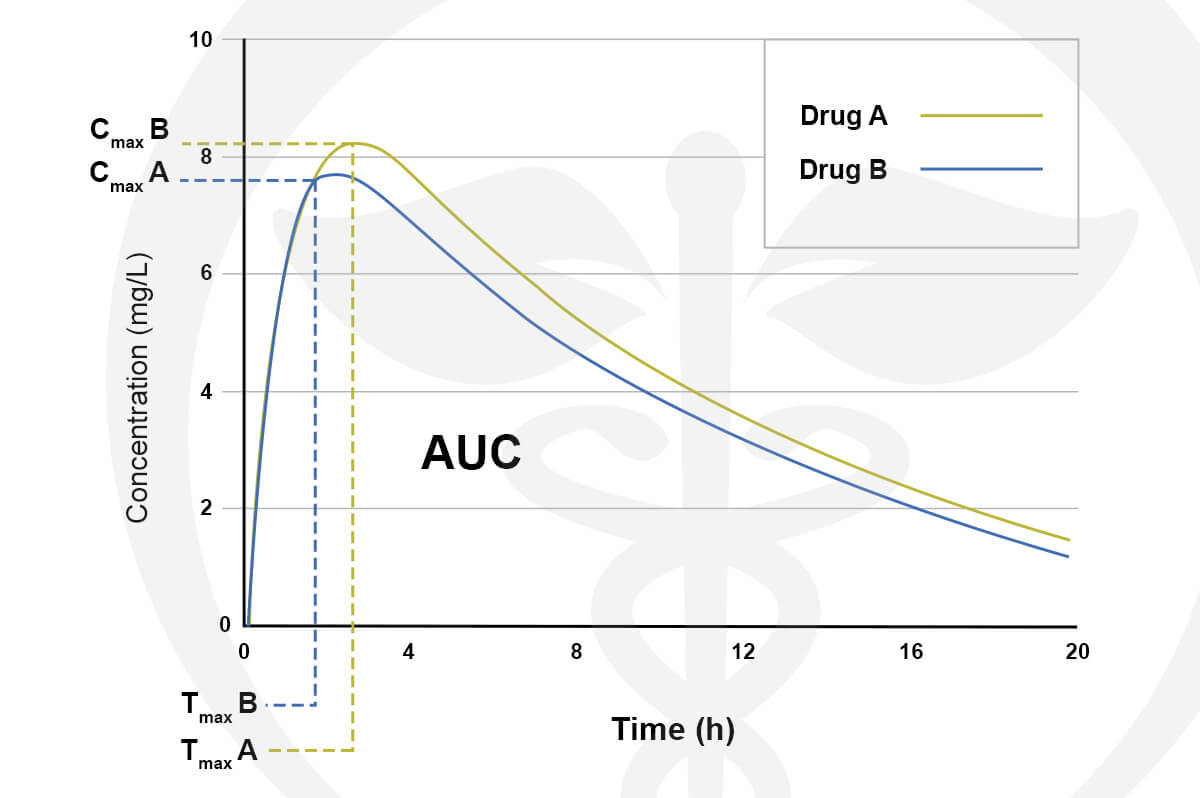
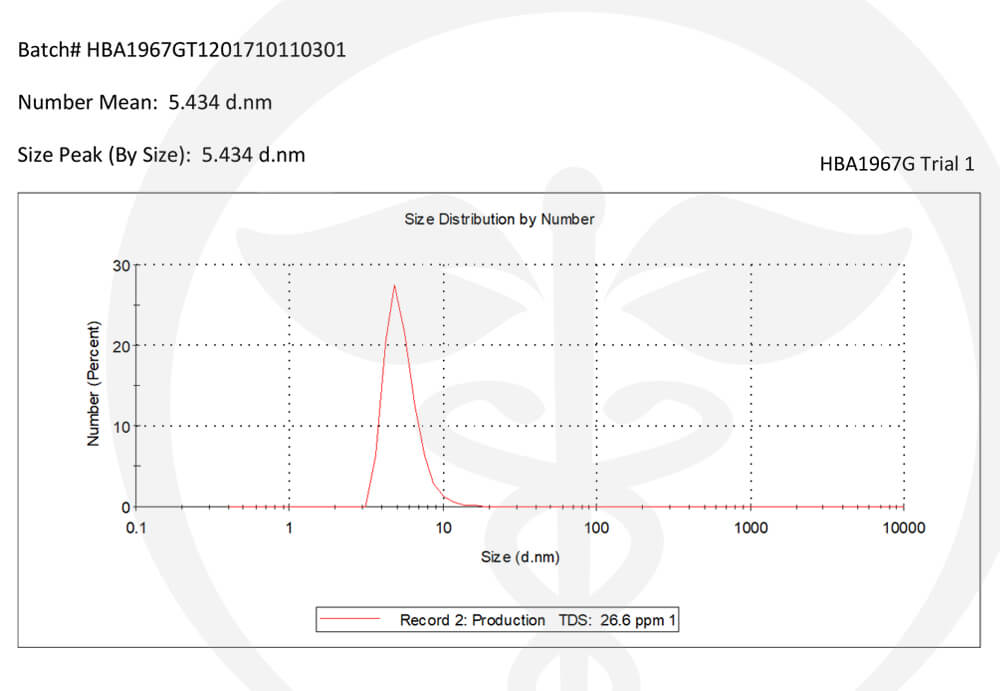
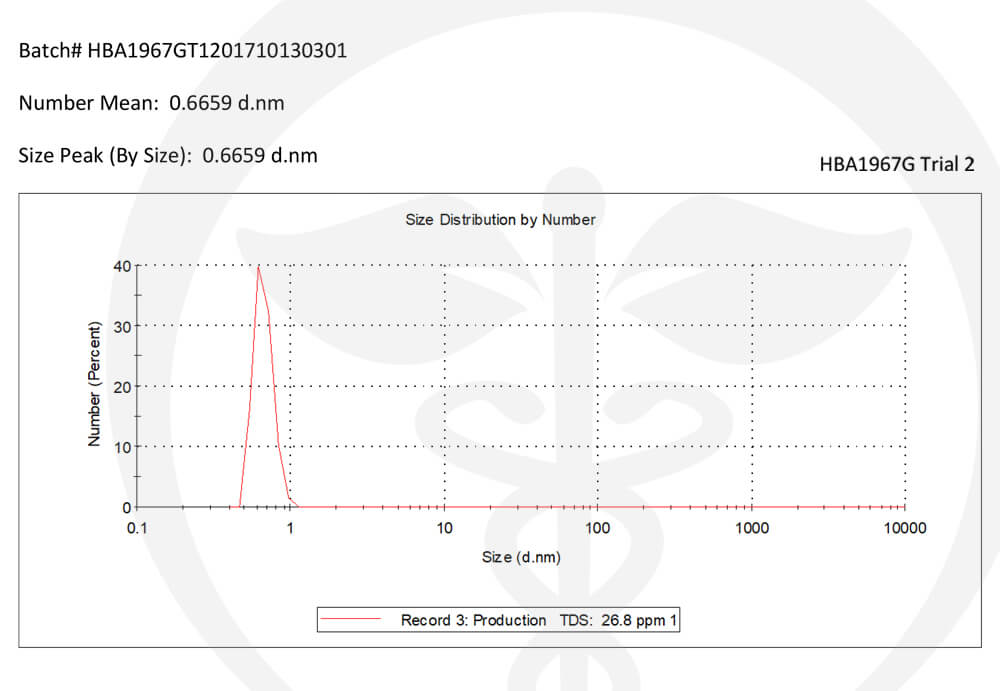
Every product we formulate is tested for potency levels to confirm that the expected cannabinoid content is, in fact, the true content. Other specialty tests and analyses are performed on unique products such as our True Nano™ water. Using a Malvern Zetasizer Non-Invasive Backscatter (NIBS), which is not commonly found in cannabis-focused testing labs, allows us to accurately measure nanoparticle size (7nm).
Potency Test Used
High-Performance Liquid Chromatography (HPLC)
Particle Test Used
Backscatter Test for True Nano Particle Size

Academic & Clinical Partnerships for
Consumer Monitoring Studies
Hemoscreen™
(Cannabinoid Blood Tests)
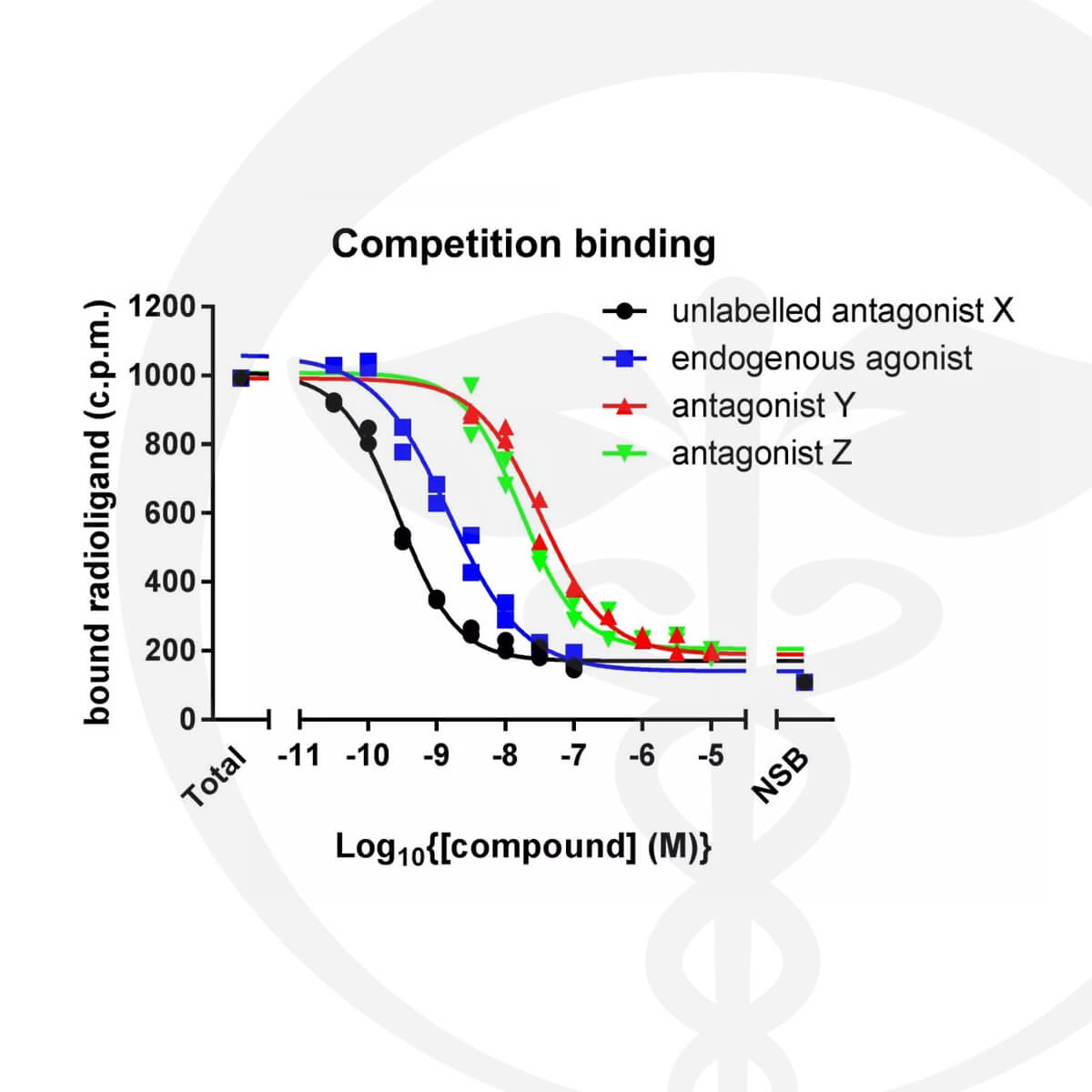
Radiobinding Assays
(CB1/CB2 Receptor Binding Affinity of Cannabinoids)
Complete supply chain transparency and product testing results available to the public